|
Structurally, the EGF domain is typically described as a small domain of 30-40 amino acids primarily stabilized by three disulfides with disulfide connectivity ababcc (1-3,2-4,5-6). The domain consists of two β-sheets, usually referred to as the major (N-terminal) and minor (C-terminal) sheets. The half-cystines of the abc motif are arranged in a triangle on the major sheet. Two different types of three-disulfide EGF domains can be differentiated that chiefly differ in the structure of the minor sheet and the location of disulfide c within it. |
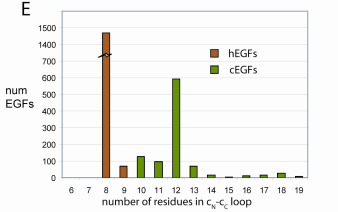 Distribution of disulfide c loop lengths in EGF modules in Swiss-Prot 43. The bimodal distribution of loop lengths is due to the presence of two different EGF type. |
What are the different types?
What type of EGF domain is my protein?
Search Swiss-Prot
Read more about it (link to journal)