At the sequence level, the two major types of three-disulfide EGFs can be differentiated by a number of features. hEGF subunits almost always have eight residues between the two half-cystines of disulfide c. The integrin β-4 subunit and prostaglandin H-synthase are the only structurally characterized hEGF subunits that do not comply with this rule, having nine residues instead. cEGF subunits typically have 10-13 residues separating the half-cystines of disulfide c.
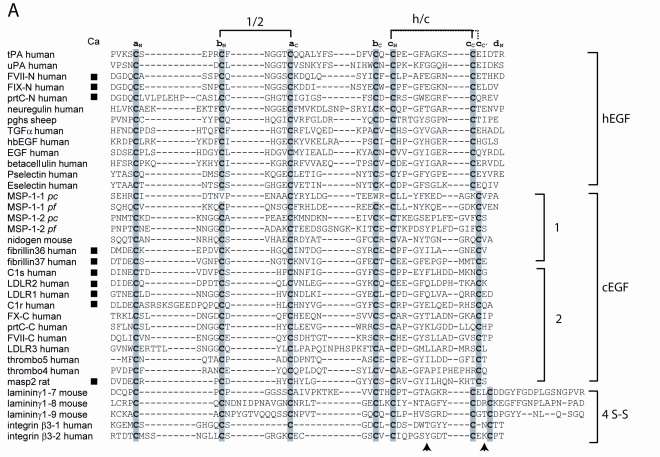
Structure-based alignment of EGF domains showing the three different three-disulfide EGF subtypes. The cN−cC loop discriminates between the hEGF and cEGF groups. Residue discriminators include cN+6 residue which is a Gly in hEGF subunits. Residues which are conserved between hEGFs and cEGFs are the cN+3 residue which is generally Gly, and the cN+4 residue which is often F/Y.