|
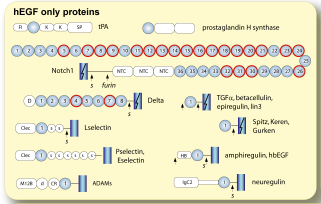
Important post-translational modifications of 3-disulfide EGFs, including unusual forms of glycosylation and posttranslational proteolytic processing, are dependent on EGF subtype. For example, EGF domains that are shed from the cell surface and mediate intercellular signaling are all hEGFs, as are all human EGF receptor family ligands. |
 |
Summary of known functional differences between 3-disulphide EGF types
| hEGF type | cEGF type |
|---|---|
|
|
|
|
|
|
|
Examples of functional differences
Arrangement of different types in mosaic proteins
Functionally distinct domains of LTBP-1 contain distinct EGF types
Differential glycosylation and hydroxylation of EGF types