|
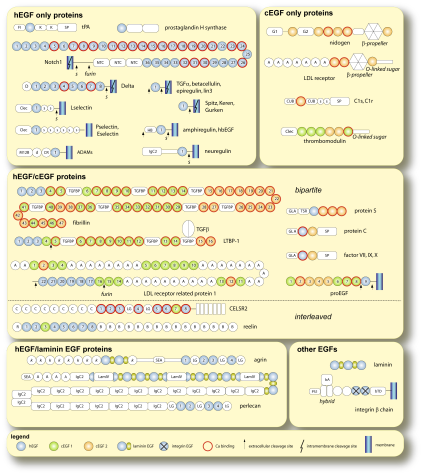 Many mosaic proteins are homogeneous with respect to EGF type. For example, many developmentally important proteins such as Notch and Delta as well as EGFs that are mitogenic contain only hEGFs. Proteins that contain solely cEGFs on the other hand include thrombomodulin and the LDL receptor. However, there are a significant number of mosaic proteins that contain both types. For these mixed EGF proteins, a bipartite structure where the different EGF types are grouped together is the most common, but other interleaved arrangements are also found. For example, most of the proteins involved in blood coagulation are a mixture of hEGFs and cEGFs with the hEGF always N-terminal to the cEGF. Fibrillin and LTBP-1,components of the extracellular matrix (ECM), have a similar arrangement. They predominantly consist of the cEGF type but are predicted to have one to three hEGFs at the N-terminus. In contrast, the LDL receptor-related protein 1 (LRP1), nidogen, the transmembrane receptor adhesion protein MUA3, and proEGF also contain predominantly cEGFs but have the opposite arrangement, with the few hEGF subunits disposed towards the C-terminus near the membrane. In addition, mosaic proteins that contain laminin EGFs contain hEGFs only. Perlecan and agrin are examples.
Many mosaic proteins are homogeneous with respect to EGF type. For example, many developmentally important proteins such as Notch and Delta as well as EGFs that are mitogenic contain only hEGFs. Proteins that contain solely cEGFs on the other hand include thrombomodulin and the LDL receptor. However, there are a significant number of mosaic proteins that contain both types. For these mixed EGF proteins, a bipartite structure where the different EGF types are grouped together is the most common, but other interleaved arrangements are also found. For example, most of the proteins involved in blood coagulation are a mixture of hEGFs and cEGFs with the hEGF always N-terminal to the cEGF. Fibrillin and LTBP-1,components of the extracellular matrix (ECM), have a similar arrangement. They predominantly consist of the cEGF type but are predicted to have one to three hEGFs at the N-terminus. In contrast, the LDL receptor-related protein 1 (LRP1), nidogen, the transmembrane receptor adhesion protein MUA3, and proEGF also contain predominantly cEGFs but have the opposite arrangement, with the few hEGF subunits disposed towards the C-terminus near the membrane. In addition, mosaic proteins that contain laminin EGFs contain hEGFs only. Perlecan and agrin are examples.
|