|
Disulfides are generally viewed as structurally stabilizing elements in proteins. However, emerging evidence shows that a subclass of disulfides in proteins may act as redox-controlled switches governing conformational changes in proteins. We believe these disulfides are distinguished from the more conventional structural stabilizers by their high torsional energies and their availability or potential availability to solvent. We first unearthed examples of these high torsional energy disulfides during a structural data mining survey of Β-sheets (Wouters and Curmi, 1995) which revealed disulfides linked across the strands. These cross-strand disulfides (CSDs) attracted our notice because they occur in a secondary structure that is already non-covalently linked and because it had previously been asserted that such structures would not exist because of the incompatible conformational constraints of Β-sheets and disulfides (Richardson, 1981). Indeed, we found the disulfides and the Β-sheets around them were significantly distorted. Recently, it was shown that cleavage of a CSD in CD4 is required for entry of HIV-1 into the cell (Matthias et al, 2002). We have recently reviewed evidence for the role of one class of these disulfides (cross-strand disulfides) in the cell entry events mediated by viral envelope proteins and bacterial toxins (Wouters, Lau & Hogg, 2004). Structural data mining of the PDB done to date suggests that in addition to cell entry mediated by exogeneous organisms, redox controlled nanomachines may contribute to other cellular processes such as signal transduction, amyloid formation and enzyme inhibition. We are currently further investigating these unusual disulfides. |
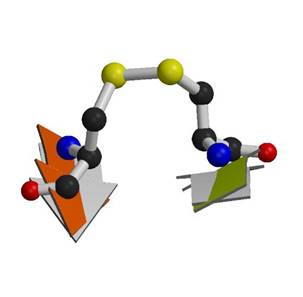
Figure 1. A typical cross-strand disulfide in botulinum neurotoxin A. Linkage of the disulfide between the Β strands is accomplished by distorting the Β sheet so that the strands are tilted towards each other.The distortion imparts a high torsional energy on the bond. The grey strands are a typical, locally flat Β-sheet from CD4 shown for comparison. The green strand in neurotoxin A is tilted towards the orange strand by ~30° with respect to the aligned strand in CD4, while the orange strand is tilted towards the green with respect to CD4 by ~15°.. |
 |
 |
 |
| ARSC (1 MB) | CLIC1 (1.7 MB) | CPDase (0.9 MB) |
|
Richardson JS. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981; 34: 167-339. Link PubMed Wouters MA, Lau KK, Hogg PJ. Cross-strand disulphides in cell entry proteins: poised to act. Bioessays. 2004 Jan; 26(1): 73-79. Download PDF PubMed Wouters MA, Curmi PM. An analysis of side chain interactions and pair correlations within antiparallel beta-sheets: the differences between backbone hydrogen-bonded and non-hydrogen-bonded residue pairs. Proteins. 1995 Jun; 22(2): 119-31. PubMed Matthias LJ, Yam PT, Jiang XM, Vandegraaff N, Li P, Poumbourios P, Donoghue N, Hogg PJ. Disulphide exchange in domain 2 of CD4 is required for entry of HIV-1. Nat Immunol. 2002 Aug; 3(8): 727-32. Epub 2002 Jul 01. PubMed |